Titration Table Example . In example 15.7.1, we calculated ph at four points during a titration. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. List the known values and plan the problem. The second example addresses a weak acid titration requiring. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Pour approximately 100cm3 of the standard solution of known concentration into a.
from www.chegg.com
List the known values and plan the problem. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Pour approximately 100cm3 of the standard solution of known concentration into a. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. In example 15.7.1, we calculated ph at four points during a titration. The second example addresses a weak acid titration requiring.
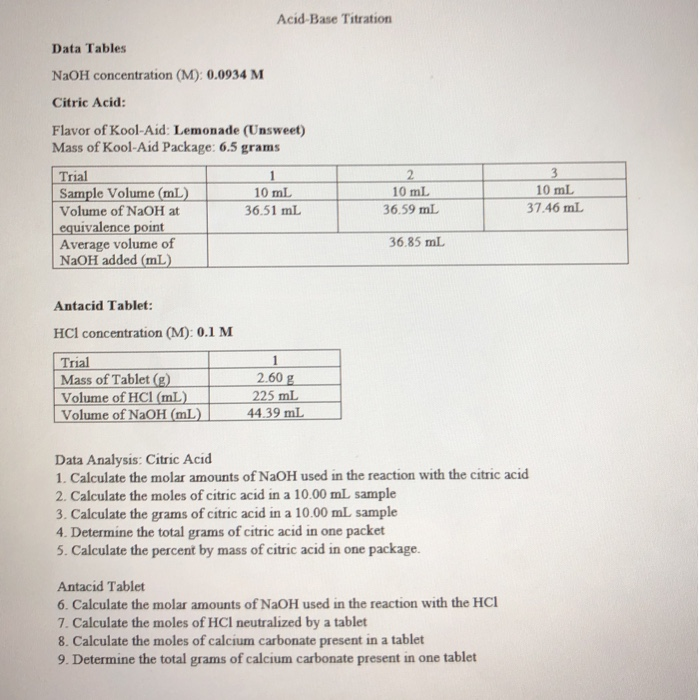
Solved AcidBase Titration Data Tables NaOH concentration
Titration Table Example The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. List the known values and plan the problem. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In example 15.7.1, we calculated ph at four points during a titration. The second example addresses a weak acid titration requiring. Pour approximately 100cm3 of the standard solution of known concentration into a.
From www.linstitute.net
IB DP Chemistry SL复习笔记1.2.9 Titrations翰林国际教育 Titration Table Example The second example addresses a weak acid titration requiring. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. Pour approximately 100cm3 of the standard solution of known concentration. Titration Table Example.
From www.numerade.com
SOLVED Table 1 Titration of 5.0 mL nonaqueous layer [Iâ‚‚] (mol/L Titration Table Example Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The second example addresses a weak. Titration Table Example.
From www.youtube.com
Back Titration Example YouTube Titration Table Example The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Pour approximately 100cm3 of the standard solution of known concentration into a. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. In example 15.7.1, we calculated ph at four. Titration Table Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Table Example The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Pour approximately 100cm3 of the standard solution of known concentration into a. List the known values and plan the problem. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. Titration is the experimental method of determining a. Titration Table Example.
From www.chegg.com
Table 2. Results of titration of EDTA with 0.025 M Titration Table Example In example 15.7.1, we calculated ph at four points during a titration. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. The first example involves a strong acid titration that requires only stoichiometric calculations to. Titration Table Example.
From www.numerade.com
SOLVED Lab Notebook Only complete the table for the successful Titration Table Example The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In example 15.7.1, we calculated ph at four points during a titration. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. List the known values and plan the problem.. Titration Table Example.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Table Example The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. In example 15.7.1, we calculated ph at four points during a titration. The following example exercise demonstrates the computation of ph. Titration Table Example.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Table Example The second example addresses a weak acid titration requiring. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the. Titration Table Example.
From studylib.net
CDCP 02.28.11 Titration Excel Practice Example Titration Table Example In example 15.7.1, we calculated ph at four points during a titration. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. Pour approximately 100cm3 of the standard solution of known concentration into a. List the known values and plan the problem. Titration is the experimental method of determining a solution of unknown concentration by the. Titration Table Example.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration Table Example In example 15.7.1, we calculated ph at four points during a titration. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. Pour approximately 100cm3 of the standard solution of known concentration into a. Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. Table 15.7.1. Titration Table Example.
From www.coursehero.com
[Solved] The table given shows data from the titration of four 10.0 mL Titration Table Example In example 15.7.1, we calculated ph at four points during a titration. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. List the known values and plan the problem. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in. Titration Table Example.
From www.studocu.com
Titration Report BTEC Applied Science Unit2 A Titration Report Titration Table Example Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. In example 15.7.1, we calculated ph at four points during a titration. Pour approximately 100cm3 of the standard solution of known concentration into a. List the known values and plan the problem. Table \(\pageindex{1}\) shows the four types of titrations,. Titration Table Example.
From www.chegg.com
Solved Data Table 2. Titration Curve values. Drops NaOH Titration Table Example Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. The second example addresses a weak acid titration requiring. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Table 15.7.1 shows a detailed sequence of changes in the ph of a. Titration Table Example.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Table Example The second example addresses a weak acid titration requiring. In example 15.7.1, we calculated ph at four points during a titration. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. Table. Titration Table Example.
From www.vrogue.co
Solved Acid Base Titrations Consider The Titration Of vrogue.co Titration Table Example Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. The second example addresses a weak acid titration requiring. Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. In example 15.7.1, we calculated ph at four points during. Titration Table Example.
From www.chegg.com
Solved Using the titration data, determine the concentration Titration Table Example The second example addresses a weak acid titration requiring. The first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. List the known values and plan the problem. Table \(\pageindex{1}\) shows the four. Titration Table Example.
From www.youtube.com
02 Experimental Set Up and Drawing Titration Table YouTube Titration Table Example Table \(\pageindex{1}\) shows the four types of titrations, and you note that the titrant. Pour approximately 100cm3 of the standard solution of known concentration into a. The second example addresses a weak acid titration requiring. List the known values and plan the problem. The following example exercise demonstrates the computation of ph for a titration solution after additions of several. Titration Table Example.
From childhealthpolicy.vumc.org
⭐ Acetic acid and naoh titration. Titration of NaOH with acetic acid Titration Table Example Table 15.7.1 shows a detailed sequence of changes in the ph of a strong acid and a weak acid in a. Titration is the experimental method of determining a solution of unknown concentration by the controlled addition of some reactant that,. List the known values and plan the problem. The first example involves a strong acid titration that requires only. Titration Table Example.